-



-
Small Molecule Drug Platforms

-
Chiral Catalysis Technology
-
Continuous Flow Technology
-
Fluorine Chemistry
-
Enzyme Catalysis
-
Photocatalysis
-
Solid-State Research
显示更多 -
-
Drug Product (DP) Platforms

-
Sustained and Controlled Release
-
Solubility Enhancement
-
Topical Formulation
-
Liquid Formulation
-
Softgels
-
Oral Fast Dissolving Technology
显示更多 -
-
Peptide Platforms
 显示更多
显示更多 -
Research Team
-
Service Platform
Related Services and Solutions

We look forward to working with you and providing you with excellent service.
DP Production Base — Taizhou Biopharma
Taizhou Biopharma supports end-to-end DP R&D and production from preclinical development, registration batch manufacturing, to commercial production.

Floor area:Approx. 120,000 m2
Quality Management:Establishing a comprehensive quality management system and processes, committed to continuously meeting the relevant requirements of China GMP, EU GMP, and FDA CGMP. We have successfully passed the registration site inspections and GMP compliance checks by the national and provincial authorities, as well as the GMP compliance inspection by the UK Medicines and Healthcare products Regulatory Agency.
Dosage forms:We plan to produce a wide range of dosage forms, including solid and liquid dosage forms and powders for injection.
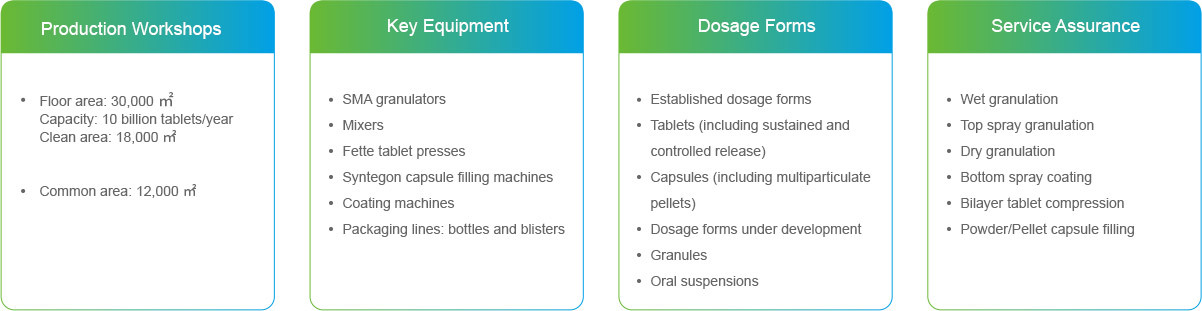
Production Equipment

Business Collaboration
Innovative Drug CDMO Services
Tel: 86-576-85588039 / 86-576-85580090
E-mail: bd@jiuzhoupharma.com

COOKIES
Our website uses cookies and similar technologies to personalize the advertising shown to you and to help you get the best experience on our website. For more information, see our Privacy & Cookie Policy
COOKIES
Our website uses cookies and similar technologies to personalize the advertising shown to you and to help you get the best experience on our website. For more information, see our Privacy & Cookie Policy
These cookies are necessary for basic functions such as payment. Standard cookies cannot be turned off and do not store any of your information.
These cookies collect information, such as how many people are using our site or which pages are popular, to help us improve the customer experience. Turning these cookies off will mean we can't collect information to improve your experience.
These cookies enable the website to provide enhanced functionality and personalization. They may be set by us or by third-party providers whose services we have added to our pages. If you do not allow these cookies, some or all of these services may not function properly.
These cookies help us understand what you are interested in so that we can show you relevant advertising on other websites. Turning these cookies off will mean we are unable to show you any personalized advertising.



