-



-
Small Molecule Drug Platforms

-
Chiral Catalysis Technology
-
Continuous Flow Technology
-
Fluorine Chemistry
-
Enzyme Catalysis
-
Photocatalysis
-
Solid-State Research
显示更多 -
-
Drug Product (DP) Platforms

-
Sustained and Controlled Release
-
Solubility Enhancement
-
Topical Formulation
-
Liquid Formulation
-
Softgels
-
Oral Fast Dissolving Technology
显示更多 -
-
Peptide Platforms
 显示更多
显示更多 -
Research Team
-
Service Platform
Related Services and Solutions

We look forward to working with you and providing you with excellent service.
Peptide CDMO Services
Jiuzhou Pharma's peptides platform provides one-stop CDMO services to global clients, from preclinical pharmaceutical research to commercial manufacturing of peptide (including conjugated) APIs.

Highlights
The Suzhou Production Base has a GMP peptide manufacturing workshop with a production capacity of 3 kg/batch and 70–90 kg/year of peptide APIs.
The Raybow Production Base in Zhejiang has OEB 4 and OEB 5 GMP production workshops for highly potent drug conjugates, with a production capacity of 600 g/batch and 25–30 kg/year.
Peptide Platform Equipment

Hangzhou Laboratory

Suzhou GMP Workshop

Taizhou High Potency Manufacturing Facility
Oligonucleotide Platform Equipment Overview

Purification System (Sepure SDL100

Tangential Flow Filtration System ( inscinstech TFF)
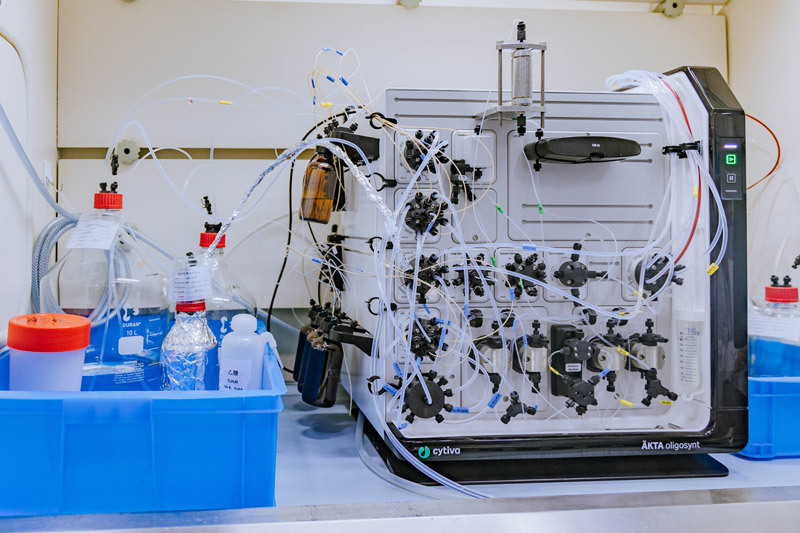
oligonucleotide synthesizer(cytiva oligosynt)

oligonucleotide synthesizer (K&A H-8)
Business Collaboration
Innovative Drug CDMO Services
Tel: 86-576-85588039 / 86-576-85580090
E-mail: bd@jiuzhoupharma.com

COOKIES
Our website uses cookies and similar technologies to personalize the advertising shown to you and to help you get the best experience on our website. For more information, see our Privacy & Cookie Policy
COOKIES
Our website uses cookies and similar technologies to personalize the advertising shown to you and to help you get the best experience on our website. For more information, see our Privacy & Cookie Policy
These cookies are necessary for basic functions such as payment. Standard cookies cannot be turned off and do not store any of your information.
These cookies collect information, such as how many people are using our site or which pages are popular, to help us improve the customer experience. Turning these cookies off will mean we can't collect information to improve your experience.
These cookies enable the website to provide enhanced functionality and personalization. They may be set by us or by third-party providers whose services we have added to our pages. If you do not allow these cookies, some or all of these services may not function properly.
These cookies help us understand what you are interested in so that we can show you relevant advertising on other websites. Turning these cookies off will mean we are unable to show you any personalized advertising.



